|
|
Posted in Crop protection | Leave a Comment »
/ By Jennifer Nichols
Posted 4h ago4 hours ago
abc.net.au/news/integrated-pest-management-beneficial-insects-horticulture/103763226Copy link
Link copied Share article
As concern over chemical use in food production grows and insect species become more resistant to poisons, farmers are turning to nature for solutions to pests that can cripple crop production.
Billions of tiny, blind, predatory mites are being bred, harvested, packed on ice, and posted to strawberry farms in the battle against destructive sap-sucking insects.
“We’re producing beneficial insects for farmers to use instead of insecticides,” Bugs for Bugs Donnybrook insectarium manager James Hill said.
“Ninety per cent of farmers in the strawberry industry are using our product.”
Australians love strawberries — 72 per cent of households bought them last financial year and on average we each ate around 2.27 kilograms of the fruit.
One of the main insect enemies that farmers battle to produce tasty red strawberries is two-spotted mites, a sap-sucking species related to ticks, too tiny to spot with the naked eye.
Left unchecked, you can see the damage two-spotted mites can do, sucking the life out of bean leaves in the polytunnels where they are raised as food for the predator mites that are sold to growers.
“If left unchecked the two-spotted mite would just devastate your crop, it would wipe you out,” Queensland Strawberry Growers president Adrian Schultz said.
An eight-legged member of the arachnid family, Phytoseiulus persimilis, is blind.
It hunts down two-spotted mites by touch and scent and can be dropped by drone to decimate populations of two-spotted mites and spider mites.
Just 0.5mm long, persimilis are voracious, specialised predators that breed twice as fast as their prey, can be carried on the wind, and are deployed to protect crops, greenhouses and commercial installations of indoor plants.
Once they have exterminated the pests they turn on their own eggs and larvae, posing no threat to other insects.
Mr Shultz said the insects have become real cost savers for big farms.
https://www.youtube.com/embed/jE8xHOTCfGA?feature=oembedYOUTUBENathan Roy’s drone dropping beneficial bugs.
“In years gone by, we had to rotate different insecticides to control the two-spotted mite and you’d get a higher percentage of pests that were resistant,” he said.
“The advent of the predator mites enabled industry to use considerably less chemicals in controlling pests, now we also have the option of introducing lady beetles into our crops to control aphids.”
Integrated pest management (IPM) is increasingly popular with farmers and uses a range of preventive measures to control pests, including natural predators, parasites, nematodes, and pheromone traps.
“It’s not set and forget, you need to monitor the situation and you’ve got to be aware of the impacts of environmental conditions,” Mr Schultz said.
The job satisfaction of helping farmers produce higher quality products with fewer chemicals is why Bugs for Bugs director Paul Jones has been working in integrated pest management for 30 years.
“When we first went out to farmers there was a lot of fear and scepticism about reducing the use of sprays and using beneficial insects to control pests,” the agricultural scientist said.
“The change has been quite radical, what was once considered a cottage industry for small organic and family farms has now become the backbone for pest management in conventional agriculture.”
https://www.youtube.com/embed/8sEVXfjX3s8?feature=oembedYOUTUBEUsing good bugs to fight bad bugs could be the key to pesticide-free farming
Bugs for Bugs is one of only a handful of commercial suppliers of beneficial insects in Australia.
From insectaries at Donnybrook, Toowoomba and Mundubbera, it sells 12 different species including predatory mites, ladybirds, lacewings, and parasitic wasps.
Home gardeners can also order the insects online.
Worldwide, predatory bioagents are being used to target gnats, thrips, caterpillars, scale, mealybugs, aphids, heliothis larvae, loopers, whitefly, and mites in crops including strawberries, raspberries, blackberries, cotton, macadamias, almonds, avocados, citrus, maize, cut flowers and hops.
Parasitic wasps kill fly maggots for the poultry, pig, dairy and feedlot industries, and black soldier fly larvae transform organic waste into compost.
At the Donnybrook insectary, billions of persimilis are being harvested from polytunnels for the start of the Queensland winter strawberry season.
Each insect order is weighed and packed on ice to keep the persimilis mites in hibernation during transport.
A vermiculite mineral is included to make it easier for farmers to evenly spread the tiny predators on their fields.
“It’s evolved, refining the craft, we’ve got better and better,” Mr Jones said.
“Mainstream chemical companies now collaborate with us to ensure products are less harmful to beneficial insects.”
More on:
Posted in Biological control, insecticides, Natural enemies | Tagged gardening, pest control, pests, plants | Leave a Comment »
The Conversation
Published: March 22, 2024 8:34am EDT
Matt Kasson receives funding from the US Department of Agriculture.
West Virginia University provides funding as a member of The Conversation US.
 We believe in the free flow of information
We believe in the free flow of informationRepublish this article
With the arrival of spring in North America, many people are gravitating to the gardening and landscaping section of home improvement stores, where displays are overstocked with eye-catching seed packs and benches are filled with potted annuals and perennials.
But some plants that once thrived in your yard may not flourish there now. To understand why, look to the U.S. Department of Agriculture’s recent update of its plant hardiness zone map, which has long helped gardeners and growers figure out which plants are most likely to thrive in a given location.
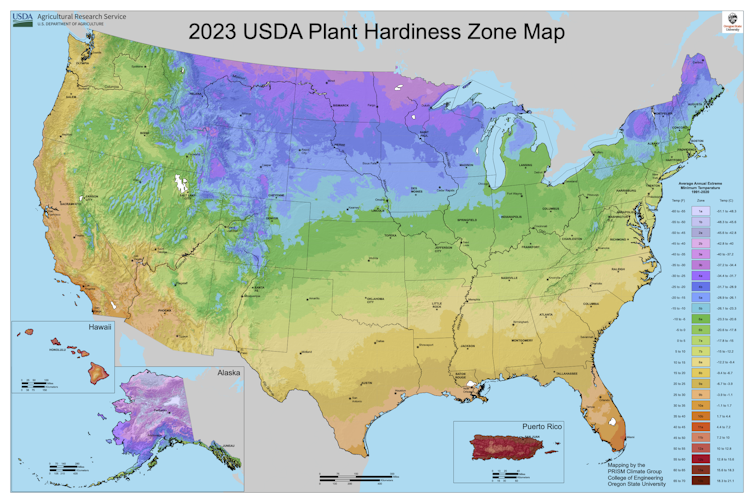
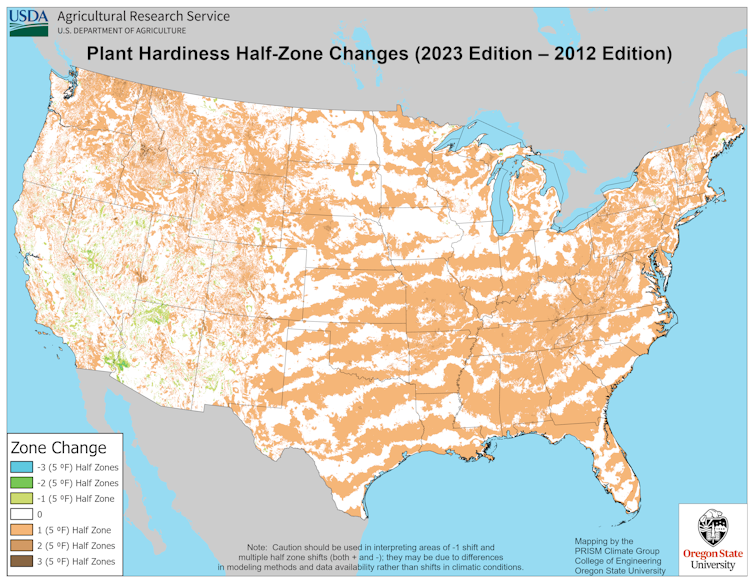
Comparing the 2023 map to the previous version from 2012 clearly shows that as climate change warms the Earth, plant hardiness zones are shifting northward. On average, the coldest days of winter in our current climate, based on temperature records from 1991 through 2020, are 5 degrees Fahrenheit (2.8 Celsius) warmer than they were between 1976 and 2005.
In some areas, including the central Appalachians, northern New England and north central Idaho, winter temperatures have warmed by 1.5 hardiness zones – 15 degrees F (8.3 C) – over the same 30-year window. This warming changes the zones in which plants, whether annual or perennial, will ultimately succeed in a climate on the move.
As a plant pathologist, I have devoted my career to understanding and addressing plant health issues. Many stresses not only shorten the lives of plants, but also affect their growth and productivity.
I am also a gardener who has seen firsthand how warming temperatures, pests and disease affect my annual harvest. By understanding climate change impacts on plant communities, you can help your garden reach its full potential in a warming world.
There’s no question that the temperature trend is upward. From 2014 through 2023, the world experienced the 10 hottest summers ever recorded in 174 years of climate data. Just a few months of sweltering, unrelenting heat can significantly affect plant health, especially cool-season garden crops like broccoli, carrots, radishes and kale.

Winters are also warming, and this matters for plants. The USDA defines plant hardiness zones based on the coldest average annual temperature in winter at a given location. Each zone represents a 10-degree F range, with zones numbered from 1 (coldest) to 13 (warmest). Zones are divided into 5-degree F half zones, which are lettered “a” (northern) or “b” (southern).
For example, the coldest hardiness zone in the lower 48 states on the new map, 3a, covers small pockets in the northernmost parts of Minnesota and has winter extreme temperatures of -40 F to -35 F. The warmest zone, 11b, is in Key West, Florida, where the coldest annual lows range from 45 F to 50 F.
On the 2012 map, northern Minnesota had a much more extensive and continuous zone 3a. North Dakota also had areas designated in this same zone, but those regions now have shifted completely into Canada. Zone 10b once covered the southern tip of mainland Florida, including Miami and Fort Lauderdale, but has now been pushed northward by a rapidly encroaching zone 11a.
Many people buy seeds or seedlings without thinking about hardiness zones, planting dates or disease risks. But when plants have to contend with temperature shifts, heat stress and disease, they will eventually struggle to survive in areas where they once thrived.
Successful gardening is still possible, though. Here are some things to consider before you plant:
Hardiness zones matter far less for annual plants, which germinate, flower and die in a single growing season, than for perennial plants that last for several years. Annuals typically avoid the lethal winter temperatures that define plant hardiness zones.
In fact, most annual seed packs don’t even list the plants’ hardiness zones. Instead, they provide sowing date guidelines by geographic region. It’s still important to follow those dates, which help ensure that frost-tender crops are not planted too early and that cool-season crops are not harvested too late in the year.

Many perennials can grow across wide temperature ranges. For example, hardy fig and hardy kiwifruit grow well in zones 4-8, an area that includes most of the Northeast, Midwest and Plains states. Raspberries are hardy in zones 3-9, and blackberries are hardy in zones 5-9. This eliminates a lot of guesswork for most gardeners, since a majority of U.S. states are dominated by two or more of these zones.
Nevertheless, it’s important to pay attention to plant tags to avoid selecting a variety or cultivar with a restricted hardiness zone over another with greater flexibility. Also, pay attention to instructions about proper sun exposure and planting dates after the last frost in your area.
Fruit trees have two parts, the rootstock and the scion wood, that are grafted together to form a single tree. Rootstocks, which consist mainly of a root system, determine the tree’s size, timing of flowering and tolerance of soil-dwelling pests and pathogens. Scion wood, which supports the flowers and fruit, determines the fruit variety.
Most commercially available fruit trees can tolerate a wide range of hardiness zones. However, stone fruits like peaches, plums and cherries are more sensitive to temperature fluctuations within those zones – particularly abrupt swings in winter temperatures that create unpredictable freeze-thaw events.

These seesaw weather episodes affect all types of fruit trees, but stone fruits appear to be more susceptible, possibly because they flower earlier in spring, have fewer hardy rootstock options, or have bark characteristics that make them more vulnerable to winter injury.
Perennial plants’ hardiness increases through the seasons in a process called hardening off, which conditions them for harsher temperatures, moisture loss in sun and wind, and full sun exposure. But a too-sudden autumn temperature drop can cause plants to die back in winter, an event known as winter kill. Similarly, a sudden spring temperature spike can lead to premature flowering and subsequent frost kill.
Plants aren’t the only organisms constrained by temperature. With milder winters, southern insect pests and plant pathogens are expanding their ranges northward.
One example is Southern blight, a stem and root rot disease that affects 500 plant species and is caused by a fungus, Agroathelia rolfsii. It’s often thought of as affecting hot Southern gardens, but has become more commonplace recently in the Northeast U.S. on tomatoes, pumpkins and squash, and other crops, including apples in Pennsylvania.

Other plant pathogens may take advantage of milder winter temperatures, which leads to prolonged saturation of soils instead of freezing. Both plants and microbes are less active when soil is frozen, but in wet soil, microbes have an opportunity to colonize dormant perennial plant roots, leading to more disease.
It can be challenging to accept that climate change is stressing some of your garden favorites, but there are thousands of varieties of plants to suit both your interests and your hardiness zone. Growing plants is an opportunity to admire their flexibility and the features that enable many of them to thrive in a world of change.
Information is flying at us from all directions. And it can be overwhelming. Wouldn’t it be easier if you could get trusted science information in one place? That place is The Conversation. As an editor here, I am fortunate to work with scientists and researchers who explain their latest research. And each week, our team sends an email that brings together the best of our coverage of science, technology and environment.
Subscribe

Vivian Lam
Associate Health and Biomedicine Editor




Copyright © 2010–2024, The Conversation US, Inc.
Posted in Climate change | Tagged climate, flowers, gardening, plants | Leave a Comment »
Saturday, 23 March 2024 08:16:28
PestNet
Submission
A single laccase acts as a key component of environmental sensing in a broad host range fungal pathogen
Nature
Communications Biology volume 7, Article number: 348 (2024)
Abstract
Secreted laccases are important enzymes on a broad ecological scale for their role in mediating plant-microbe interactions, but within ascomycete fungi these enzymes have been primarily associated with melanin biosynthesis. In this study, a putatively secreted laccase, Sslac2, was characterized from the broad-host-range plant pathogen Sclerotinia sclerotiorum, which is largely unpigmented and is not dependent on melanogenesis for plant infection. Gene knockouts of Sslac2 demonstrate wide ranging developmental phenotypes and are functionally non-pathogenic. These mutants also displayed indiscriminate growth behaviors and enhanced biomass formation, seemingly as a result of their inability to respond to canonical environmental growth cues, a phenomenon further confirmed through chemical stress, physiological, and transcriptomic analyses. Transmission and scanning electron microscopy demonstrate apparent differences in extracellular matrix structure between WT and mutant strains that likely explain the inability of the mutants to respond to their environment. Targeting Sslac2 using host-induced gene silencing significantly improved resistance to S. sclerotiorum, suggesting that fungal laccases could be a valuable target of disease control. Collectively, we identified a laccase critical to the development and virulence of the broad-host-range pathogen S. sclerotiorum and propose a potentially novel role for fungal laccases in modulating environmental sensing.
Read on: https://www.nature.com/articles/s42003-024-06034-7
Saturday, 23 March 2024 08:16:28
Submission
A single laccase acts as a key component of environmental sensing in a
broad host range fungal pathogen
Nature
Communications
Biology volume 7,
Article number: 348 (2024)
Abstract
Secreted laccases are important enzymes on a broad ecological scale for
their role in mediating plant-microbe interactions, but within ascomycete fungi
these enzymes have been primarily associated with melanin biosynthesis. In this
study, a putatively secreted laccase, Sslac2, was characterized
from the broad-host-range plant pathogen Sclerotinia sclerotiorum,
which is largely unpigmented and is not dependent on melanogenesis for plant
infection. Gene knockouts of Sslac2 demonstrate wide ranging
developmental phenotypes and are functionally non-pathogenic. These mutants
also displayed indiscriminate growth behaviors and enhanced biomass formation,
seemingly as a result of their inability to respond to canonical environmental
growth cues, a phenomenon further confirmed through chemical stress,
physiological, and transcriptomic analyses. Transmission and scanning electron
microscopy demonstrate apparent differences in extracellular matrix structure
between WT and mutant strains that likely explain the inability of the mutants
to respond to their environment. Targeting Sslac2 using
host-induced gene silencing significantly improved resistance to S.
sclerotiorum, suggesting that fungal laccases could be a valuable target of
disease control. Collectively, we identified a laccase critical to the
development and virulence of the broad-host-range pathogen S.
sclerotiorum and propose a potentially novel role for fungal laccases
in modulating environmental sensing.
Read on: https://www.nature.com/articles/s42003-024-06034-7
Posted in Fungi, Plant Pathogens | Tagged fungi, science | Leave a Comment »
Harvest Public Media | By Celia Hack
Published April 8, 2024 at 4:00 AM CDT
LISTEN • 3:20

Three yellow, bug-like creatures crawl in perfectly straight lines across the dead grass of a flat, brown February field in Cheney, Kansas.
These are the namesake of GreenField Robotics. Two lights peer out from each side of the boxy machines, almost appearing like eyes. Blades whir at their base, about a half an inch from the ground – the perfect height to chop weeds, though there’s nothing to cut down on a frigid winter day.
They stick out in an otherwise rural landscape – and GreenField CEO Clint Brauer said he frequently hears from curious passersby.
“All the time,” Brauer said. “I’m always surprised, though, how little people notice.”
Brauer founded the company in 2018. The start-up has now grown large enough to attract investment from Chipotle’s $100 million venture capital fund and to secure partnerships with dog food and baking mix brands.
Brauer grew up on a family farm in Haven, Kansas, but moved to California after high school to work in the tech industry. In 2010, he returned home after his dad was diagnosed with Parkinson’s disease. He attributes the use of herbicides to his dad’s diagnosis.
“The more I learned about farm chemicals and stuff … the more I thought there’s a decent chance that this came from that,” Brauer said.

The move sucked Brauer back into the world of agriculture, where he started seeking ways to eliminate herbicides. He tried farming organically, but it was too expensive to be accessible to many buyers.
Another option was no till farming, where farmers avoid turning over the dirt to reduce erosion and improve soil health. But it’s a method that leans on herbicides.
And in 2015, Brauer was starting to notice the weeds in his fields were becoming resistant to chemicals anyway.
“There was no good way to get rid of those weeds, even though we had sprayed many times,” Brauer said.
“So, what do we do? And so that was the beginning of this idea of – what if we just cut those weeds?”
Cutting weeds by hand wasn’t exactly a 21st-century answer. So Brauer thought: What about robots? He reached out to software and machine-vision experts and started prototyping robots.
By 2021, the company had manufactured a two-and-a-half foot-tall working robot. And it pulled together different technologies, like drones, to create extremely precise maps of crop fields. The robots follow the maps, so that they’re unlikely to accidentally chop down a crop instead of a weed.
“They plant the crop, we count about 10 days, normally, the crops emerge, and we fly over it with a drone,” Brauer said. “ … That’s where AI – we have machine vision that automatically recognizes everything that’s going on in that field.”
Thirty to 40 days later, Brauer sends out the robots.

In 2022, the company partnered with MKC, a major agricultural cooperative, to reach farmers who might use the product. In 2023, GreenField Robotics worked with 25 to 30 Kansas farmers, Brauer said. The company currently has a fleet of 20 robots and 15 employees
This summer, Brauer said the company is planning to work the weed-cutting robots on over 20,000 acres.
John Niemann is a farmer in Reno County. He tested GreenField Robotics for the first time last spring on 80 acres of a sorghum field, leaving 10 acres untouched to compare results. He had treated the entire crop with herbicides earlier in the season.
“We saw higher yields where we used the robots, versus the 10 acres that we did not,” Niemann said.
That’s because the weeds that didn’t get chopped down in the 10 acres competed with the crop for moisture, hampering the yield.
“The robots are part of a toolbox, is how I would look at them,” Niemann said. “There is no magic bullet in farming practices. You need to have a lot of tools in your toolbox.”
Niemann says the robots are a useful tool to reduce reliance on chemicals. Plus, he said the cost was comparable to herbicides.
Brauer said the economics is always his first pitch to farmers, and the robots are compelling because they damage less of the crop than chemicals do.
The company is also adapting the robots for other uses, like planting cover crops and soil testing.
“We are on a mission,” Brauer said. “This is not about enrichment. This is – we’re building something that can’t be undone. And so we’re going to eliminate these chemicals.”
This story was first aired and produced by KMUW. It’s being distributed by Harvest Public Media, a collaboration of public media newsrooms in the Midwest. It reports on food systems, agriculture and rural issues.
Tags
Posted in Herbicides, Mechanical control, Weeds | Tagged agriculture, AI, farming, sustainable agriculture, technology | Leave a Comment »
Rosanna Magnano | 24 Ore | April 8, 2024


The witch hunt against old GMOs will soon be a distant memory (perhaps). And after more than twenty years of blocks, a new generation of biotech (but not transgenic) plants will take its first steps in Italy. With the last and definitive green light from the Ministry of the Environment, experimental sowing of a variety of rice in the open field was obtained thanks to the new genomic techniques, known in Italy as TEA, assisted evolution techniques.
It is a rice capable of resisting, without the use of fungicides, the attacks of the Pyricularia oryzae fungus which causes the disease commonly known as “brusone” [in Italian, or rice blast fungus in English], the most serious fungal pathology of rice which in some years can lead to production losses even of the 50%. The request for authorization for the tests was presented by the University of Milan, where the first research group in the country coordinated by the biotechnologist Vittoria Brambilla, [thanks to updated] rules for field trials of plants developed with genome editing or cisgenesis.
Follow the latest news and policy debates on sustainable agriculture, biomedicine, and other ‘disruptive’ innovations. Subscribe to our newsletter.
Laboratory tests using resistance tests have given excellent results in terms of productivity and without the administration of agrochemicals.
[Editor’s note: This article has been translated from Italian and edited for clarity.]
This is an excerpt. Read the original post here

Posted in Biotechnology, Fungi, Gene editing, Plant breeding | Tagged rice | Leave a Comment »
Tina Deines
Sun, April 7, 2024 at 12:00 PM CDT·2 min read

Researchers are exploring an exciting new approach that uses genomics to help monitor and identify pesticide resistance in the insects that munch on our crops.
Pest management is important for farmers, but insects often become immune to pesticides, making them less effective. In the new research, published in the Proceedings of the National Academy of Science, a team of scientists from the University of Maryland (UMD) presents a new strategy that analyzes genomic changes in pests to monitor and identify emerging resistance to specific toxins early on.
They zoned in on one pest in particular: the corn earworm, a crop-destroying caterpillar that has developed widespread resistance to a number of natural toxins bred into corn. They were able to identify resistance to toxins among these caterpillars after just a single generation of exposure. They also identified how common strategies for avoiding resistance could actually be doing the opposite.
“As it currently stands, the evolution of resistance across many pests of agricultural and public health importance is outpacing the rate at which we can discover new technologies to manage them,” said senior author Megan Fritz, an associate professor of entomology at UMD, per Phys.org. “I’m really excited about this study, because we’re developing the framework for use of genomic approaches to monitor and manage resistance in any system.”
The new research is one of many that is helping farmers to produce more successful harvests.
For instance, a team of American and Chinese researchers found a way to genetically engineer plants that can survive heat waves. University of Minnesota scientists are on their way to developing a “Super Grape” that could stave off powdery mildew and reduce the need for fungicide.
These developments in agriculture come at a critically important time — as our planet continues to warm, there are frequent heat waves and droughts, which threaten our food security. Plus, climate change scientists predict that a warming world will drive a surge in certain insect pests that attack our crops, further threatening food security and causing economic losses for those in the agricultural sector.
Join our free newsletter for weekly updates on the coolest innovations improving our lives and saving our planet.
Posted in Monitoring, Pesticides, Research | Tagged agriculture, environment, sustainable agriculture | Leave a Comment »
| United Kingdom – Plant breeders to benefit from online research toolsSelect April 3, 2024  An exciting new project will look to put cutting-edge research tools in the hands of plant breeders, providing access to genomic resources to accelerate the development of more resilient and climate-resistant crops.The collaboration brings together the collective expertise of the Earlham Institute, IBM Research, the Science and Technology Facilities Council (STFC) Hartree Centre, and RAGT Seeds UK with the aim of simplifying and speeding up the transition of cutting-edge genome research tools, workflows, and software into industrial applications.The one-year Excelerate project is part of the Hartree National Centre for Digital Innovation (HNCDI) programme from STFC designed to close the gap between academic and industrial applications of digital technologies – such as artificial intelligence (AI) and quantum computing.The UK is home to some of the most exciting and innovative life science research. Institutions are pioneering the use of new technologies to overcome issues of scale and complexity in data-intensive bioscience, such as developing approaches that could be used to accelerate crop breeding in line with EU safety and ethical regulations.At the Earlham Institute, this includes crop pangenomes and the tools required to analyse them, developed through its Decoding Biodiversity strategic programme – funded by the Biotechnology and Biological Sciences Research Council (BBSRC), part of UKRI.But the transition of this knowledge into usable technology – and its uptake by industry – remains a significant challenge.“Modern plant breeding practices are based on understanding and then using genetic resources – made possible by digital innovations – that breeders can incorporate into their programmes,” Professor Anthony Hall, project lead and Head of Plant Genomics at the Earlham Institute explained.“Bioinformatics and machine learning techniques are playing an increasingly important role in deciphering genetic diversity. But they bring significant overheads in terms of the bioinformatics skills and computing power required to develop and implement new workflows.”The plant breeding industry has a crucial role to play in addressing the global challenges of food security, water conservation, and net zero. To realise the enormous potential of UK science and innovation, initiatives are needed to bridge the gap between research and industry.This new project brings together leaders from academia and industry to provide cloud-based tools that can be easily adopted by the plant breeding companies to support the development of next-generation crops with greater climate resilience and improved nutritional properties.The Earlham Institute is working with IBM Research and STFC to develop new cloud-based tools – including those optimised for exploring plant pangenomes – which RAGT Seeds UK will be road testing.“The Earlham Institute is home to some amazing research infrastructure, innovation, and expertise,” says Professor Hall. “This helps us to develop the technology needed to answer the big questions that will be critical in addressing urgent global challenges, such as how we find new sources of diversity for breeding more resilient crops.”  The project team from left to right: Robin Kennedy Reid, STFC, Rachel Rusholme-Pilcher, Earlham Institute, Laura Jayne-Gardiner, IBM Research, Will Davies, STFC, Anthony Hall, Earlham Institute, Chris Burt, Heidi Town, and John Baison, RAGT Seeds UK. Dr Rachel Rusholme-Pilcher is a Senior Postdoctoral Researcher at the Earlham Institute, and has played a central role in developing the workflows that will be used in this partnership.“The tools we’re developing and optimising will allow plant breeders to interact with their complex datasets in a way they simply couldn’t before,” explained Dr Rusholme-Pilcher. “It should provide new information they can rapidly incorporate into their existing breeding programmes.“We’ll also be using this project to look at how we can embed the adoption of FAIR approaches – the movement to make all research data Findable, Accessible, Interoperable, and Reusable. Making this kind of research FAIR can be a challenge but these collaborations will hopefully change that – transforming the impact of emerging technologies.”Excelerate is one strand of a number of projects from HNCDI embedding AI solutions across UK industry. To both accelerate and simplify the adoption of compute intensive bioinformatics workflows in the plant breeding industry, this project will use an on-demand, scalable, Hybrid Cloud delivery model.Dr Laura-Jayne Gardiner, Senior Research Scientist at IBM Research, said: “Our HNCDI Excelerate projects are enabling businesses to adopt new technologies – including artificial intelligence and hybrid cloud – to overcome industrial challenges, such as allowing complex biological data analytics at increased scale and speed.”Robin Kennedy-Reid, Senior Research Software Engineer at the STFC Hartree Centre, said: “At the Hartree Centre, we use applied research and innovation to turn good ideas into industry-ready solutions for long-term societal and economic impact. This is made possible by working with a network of partners, both industry and academic leaders, as well as drawing on the work of open source communities like nf-core.“In this project, this will deliver new bioinformatics and machine learning capability to the plant breeding industry; with a view to assisting the search for more sustainable wheat varieties.”Dr John Baison, Cereals Research and Genomics Manager at RAGT Seeds UK, said: “Genome-based breeding holds promise for expediting wheat breeding, aiming to ensure sustainable wheat production by creating high-yielding, climate-resilient cultivars with superior nutritional quality.“The plant-breeding sector is positioning to confront these targets by leveraging the dynamic UK research community. However, bridging the gap between research and industry is crucial for optimising the potential of UK science and innovation.“As RAGT delves deeper into genomics activities, it has become increasingly evident that we must harness advanced computing tools to navigate the vast amounts of data generated from genetics and genomics projects. RAGT is excited to be at the forefront of this collaborative effort, which could revolutionise the application of genomics to plant breeding.” More news from: . Earlham Institute . RAGT Seeds Limited Website: http://www.earlham.ac.ukPublished: April 3, 2024 |
| The news item on this page is copyright by the organization where it originated Fair use notice |
| United Kingdom – Plant breeders to benefit from online research toolsSelect April 3, 2024  An exciting new project will look to put cutting-edge research tools in the hands of plant breeders, providing access to genomic resources to accelerate the development of more resilient and climate-resistant crops.The collaboration brings together the collective expertise of the Earlham Institute, IBM Research, the Science and Technology Facilities Council (STFC) Hartree Centre, and RAGT Seeds UK with the aim of simplifying and speeding up the transition of cutting-edge genome research tools, workflows, and software into industrial applications.The one-year Excelerate project is part of the Hartree National Centre for Digital Innovation (HNCDI) programme from STFC designed to close the gap between academic and industrial applications of digital technologies – such as artificial intelligence (AI) and quantum computing.The UK is home to some of the most exciting and innovative life science research. Institutions are pioneering the use of new technologies to overcome issues of scale and complexity in data-intensive bioscience, such as developing approaches that could be used to accelerate crop breeding in line with EU safety and ethical regulations.At the Earlham Institute, this includes crop pangenomes and the tools required to analyse them, developed through its Decoding Biodiversity strategic programme – funded by the Biotechnology and Biological Sciences Research Council (BBSRC), part of UKRI.But the transition of this knowledge into usable technology – and its uptake by industry – remains a significant challenge.“Modern plant breeding practices are based on understanding and then using genetic resources – made possible by digital innovations – that breeders can incorporate into their programmes,” Professor Anthony Hall, project lead and Head of Plant Genomics at the Earlham Institute explained.“Bioinformatics and machine learning techniques are playing an increasingly important role in deciphering genetic diversity. But they bring significant overheads in terms of the bioinformatics skills and computing power required to develop and implement new workflows.”The plant breeding industry has a crucial role to play in addressing the global challenges of food security, water conservation, and net zero. To realise the enormous potential of UK science and innovation, initiatives are needed to bridge the gap between research and industry.This new project brings together leaders from academia and industry to provide cloud-based tools that can be easily adopted by the plant breeding companies to support the development of next-generation crops with greater climate resilience and improved nutritional properties.The Earlham Institute is working with IBM Research and STFC to develop new cloud-based tools – including those optimised for exploring plant pangenomes – which RAGT Seeds UK will be road testing.“The Earlham Institute is home to some amazing research infrastructure, innovation, and expertise,” says Professor Hall. “This helps us to develop the technology needed to answer the big questions that will be critical in addressing urgent global challenges, such as how we find new sources of diversity for breeding more resilient crops.”  The project team from left to right: Robin Kennedy Reid, STFC, Rachel Rusholme-Pilcher, Earlham Institute, Laura Jayne-Gardiner, IBM Research, Will Davies, STFC, Anthony Hall, Earlham Institute, Chris Burt, Heidi Town, and John Baison, RAGT Seeds UK. Dr Rachel Rusholme-Pilcher is a Senior Postdoctoral Researcher at the Earlham Institute, and has played a central role in developing the workflows that will be used in this partnership.“The tools we’re developing and optimising will allow plant breeders to interact with their complex datasets in a way they simply couldn’t before,” explained Dr Rusholme-Pilcher. “It should provide new information they can rapidly incorporate into their existing breeding programmes.“We’ll also be using this project to look at how we can embed the adoption of FAIR approaches – the movement to make all research data Findable, Accessible, Interoperable, and Reusable. Making this kind of research FAIR can be a challenge but these collaborations will hopefully change that – transforming the impact of emerging technologies.”Excelerate is one strand of a number of projects from HNCDI embedding AI solutions across UK industry. To both accelerate and simplify the adoption of compute intensive bioinformatics workflows in the plant breeding industry, this project will use an on-demand, scalable, Hybrid Cloud delivery model.Dr Laura-Jayne Gardiner, Senior Research Scientist at IBM Research, said: “Our HNCDI Excelerate projects are enabling businesses to adopt new technologies – including artificial intelligence and hybrid cloud – to overcome industrial challenges, such as allowing complex biological data analytics at increased scale and speed.”Robin Kennedy-Reid, Senior Research Software Engineer at the STFC Hartree Centre, said: “At the Hartree Centre, we use applied research and innovation to turn good ideas into industry-ready solutions for long-term societal and economic impact. This is made possible by working with a network of partners, both industry and academic leaders, as well as drawing on the work of open source communities like nf-core.“In this project, this will deliver new bioinformatics and machine learning capability to the plant breeding industry; with a view to assisting the search for more sustainable wheat varieties.”Dr John Baison, Cereals Research and Genomics Manager at RAGT Seeds UK, said: “Genome-based breeding holds promise for expediting wheat breeding, aiming to ensure sustainable wheat production by creating high-yielding, climate-resilient cultivars with superior nutritional quality.“The plant-breeding sector is positioning to confront these targets by leveraging the dynamic UK research community. However, bridging the gap between research and industry is crucial for optimising the potential of UK science and innovation.“As RAGT delves deeper into genomics activities, it has become increasingly evident that we must harness advanced computing tools to navigate the vast amounts of data generated from genetics and genomics projects. RAGT is excited to be at the forefront of this collaborative effort, which could revolutionise the application of genomics to plant breeding.” More news from: . Earlham Institute . RAGT Seeds Limited Website: http://www.earlham.ac.ukPublished: April 3, 2024 |
| The news item on this page is copyright by the organization where it originated Fair use notice |
Posted in Host plant resistance | Tagged agriculture, genetics, Plant breeding | Leave a Comment »
By Sofia Sanchez Manzanaro | Euractiv
Est. 3min
Apr 9, 2024 (updated: Apr 10, 2024)
Content-Type: News

The Strategic Agenda, which defines the EU’s priorities for the 2024-2029 mandate and provides guidance for the Brussels-based institutions, will be adopted by the 27 heads of state and government during the European Council meeting of 27-28 June. [EPA/OLIVIER HOSLET]
Euractiv is part of the Trust Project >>>
EU leaders are expected to put food security at the heart of the bloc’s agricultural policy for the next five years, according to a leaked draft of the EU’s Strategic Agenda seen by Euractiv.
The programme defines Europe’s priorities for the 2024-2029 mandate, providing guidance to the EU institutions, and will be adopted by the 27 heads of state and government during the European Council meeting on 27-28 June.
The internal document, created on 27 March, predates the most recent exchanges between the EU leaders, and points to food security as a key priority for a “prosperous and competitive Europe,” despite the issue hardly being discussed at EU summits in recent years.
“Ensure our food security through a vibrant agriculture sector,” reads one of the bullet points of the draft outline.
The two-page text does not explicitly reference the sustainability of the agricultural sector or the protection of the environment, even though it prioritises “preparing for the new realities stemming from climate change.”
This initial draft marks a departure from the 2019 priorities, which included “promoting sustainable agriculture” and “calling on all EU countries to move forward and step up their climate action”.
In response to widespread farmer protests across the EU, the European Commission has already shelved or backtracked some of its plans to improve the sustainability of the farming sector in recent months.
Faustine Bas-Defossez, director for health, nature, and environment at the European Environmental Bureau (EEB), described the absence of sustainable agriculture in the leaked 2024 agenda as “deeply troubling”.
“By prioritising’ food security’ over sustainability in agriculture, EU leaders are ignoring the reality that climate change and natural disasters pose the greatest threats to our food security,” she warned.
A study commissioned by the European Parliament’s Agricultural Committee found that while food availability in the EU “is not generally considered to be at risk,” the bloc relies too heavily on imports from a reduced group of suppliers for animal feed and fertilisers.
According to the report, those dependencies, exacerbated by an uncertain geopolitical situation and climate change, could threaten the long-term resilience of the EU food system.
The study however also found that sustainable farming practices, such as organic agriculture and the promotion of lower consumption of animal products, could decrease the bloc’s need for imports.

The EU remains heavily reliant on animal feed and fertilisers imports from outside the bloc, as highlighted in a recent study commissioned by the European Parliament’s Agriculture Committee (AGRI).
[Edited by Angelo Di Mambro and Rajnish Singh]
Posted in Uncategorized | Tagged agriculture, Food Security | Leave a Comment »
Last week, the NZPPI Board agreed to sign the Solanaceae Readiness Operational Agreement (OA) on behalf of their Members. This agreement is a partnership between NZPPI, Vegetables NZ, Tomatoes NZ & Potatoes NZ to collaborate on projects to manage a range of diseases that have impacted Solanaceae horticulture crops in recent years.

The horticulture sectors have responded to several incursions in the past few years, including Pepino mosaic virus (PepMV) and Potato spindle tuber viroid (PSTVd), with an increasing risk of further incursions that will result in considerable costs and crop losses.
There are no confirmed projects or funding commitments in place at this stage, but discussions are underway to develop systems to avoid and manage future incursions. Plant producers play a key role in the production process and in managing these risks.
Signing the OA enables NZPPI to participate in the development of future management programmes, giving our members a say in how they are designed and implemented.
For more information:
New Zealand Plant Producers Incorporated
nzppi.co.nzPublication date: Mon 8 Apr 2024
Posted in Bacteria, Crop protection, Fungi, Nematodes, Plant Pathogens, Viruses | Tagged solanaceae | Leave a Comment »
Saturday, 09 March 2024 10:37:00
Submission
LETHAL YELLOWING, COCONUT PALM – JAMAICA
ProMED
http://www.promedmail.org
Source: Jamaica Observer [summ. Mod.DHA, edited]
https://www.jamaicaobserver.com/2024/03/05/spread-lethal-yellowing-disease-reduced-70/
Through the work of the Coconut Industry Board (CIB), Jamaica has been able to reduce the spread of the lethal yellowing disease in the coconut industry by 70%. CIB have contributed significantly through research which has allowed the development of varieties and hybrids with optimum resistance/tolerance to lethal yellowing. In addition, increased yields are obtained from these locally developed varieties that are adapting better to the climatic conditions.
Within the region, the disease was first discovered in the Cayman Islands in 1834 and was found in Jamaica in 1884. It became a real threat to Jamaica after 1961 and became even more significant in the 1970s when some 10 million trees of the ‘Jamaican Tall’ variety were destroyed. Lethal yellowing has caused severe economic losses in Jamaica.
—
Communicated by:
ProMED
[Lethal yellowing (LY) diseases of coconut and other palms are caused by phytoplasmas of the palm lethal yellowing (16SrIV; _Candidatus_ Phytoplasma palmae strains) group. A number of LY strains have been described from the Caribbean, Latin America, Africa and southern Asia. LY has seriously jeopardised coconut industries in the respective areas. LY-type diseases like Cape St Paul wilt (CSPW) in Ghana, the “maladie de Kaincope” in Togo and Awka disease (lethal decline, LD) in Nigeria, previously included in the 16SrIV group, have been reclassified as the new group 16SrXXII (Nigerian coconut lethal decline group, _Ca._ P. palmicola strains; see link below).
Symptoms include premature nut drop, blackening of inflorescences, yellowing of fronds; death of the palm usually occurs within 4 to 6 months. The planthopper _Myndus crudus_ is suspected to be the vector in the Americas, but different vectors may be involved in the spread of LY strains elsewhere. Seed transmission of the pathogens cannot be excluded; some weed species may serve as pathogen reservoirs. Jumps of LY across apparently unaffected coconut populations have been observed, possibly due to aerial spread of infectious vector insects or human activities. Even with strict controls, including certification of nuts and their parent trees, excluding infectious vector insects requires large quarantine efforts.
While LY affects many palm species, at least for coconut palm susceptibility may vary between cultivars or even within cultivars, depending on the region where they grow. Symptoms can be suppressed by tetracycline treatments, usually applied as trunk injections. The antibiotic inhibits multiplication of the pathogens but does not eliminate them. Therefore, treatments need to be repeated regularly. Commercial control of the diseases mostly relies on phytosanitation followed by replanting with resistant varieties.
An unexplained resistance breakdown of some widely used hybrids occurred earlier in Jamaica (ProMED post 20070522.1643) and caused great concern.
Pictures
LY symptoms on coconut and other palms:
https://www.growables.org/information/TropicalFruit/images/LethalYellowingFoliarSymptoms.jpg,
https://bugwoodcloud.org/images/768×512/1504008.jpg,
https://guyanachronicle.com/wp-content/uploads/2017/04/Lethal-Yellowing.jpg (leaf) and
http://www.cphdforum.org/wp-content/uploads/2015/06/LethalYellowingCoconutSymptom.jpg (fruit)
_Myndus crudus_:
https://bugwoodcloud.org/images/768×512/0725079.jpg
Links
Story also at:
https://jis.gov.jm/spread-of-lethal-yellowing-disease-reduced-by-70/ and
https://jamaica-gleaner.com/article/news/20240304/spread-lethal-yellowing-disease-coconut-industry-reduced-70
Lethal yellowing information:
https://doi.org/10.1079/cabicompendium.38647,
https://doi.org/10.3389/fpls.2016.01521,
https://doi.org/10.1111/j.1744-7348.2011.00480.x,
https://www.cphdforum.org/index.php/2015/06/03/about-lethal-yellowing-of-coconut/,
http://edis.ifas.ufl.edu/pp146 and
https://www.apsnet.org/edcenter/disandpath/prokaryote/pdlessons/Pages/LethalYellowing.aspx
16SrIV LY phytoplasma group taxonomy and species list:
https://www.uniprot.org/taxonomy/85624
16SrXXII classification of some LY-type diseases:
https://doi.org/10.1099/ijs.0.65000-0
16SrXXII LDN phytoplasma group taxonomy:
https://www.uniprot.org/taxonomy/590462
Phytoplasma resource centre:
https://plantpathology.ba.ars.usda.gov/phytoplasma.html
Information on LY vectors via:
https://bugguide.net/node/view/63
– Mod.DHA
Posted in Crop protection, Emerging/invasive pests, Host plant resistance, insecticides, Phytoplasma, Phytosanitary | Tagged disease, jamaica | Leave a Comment »